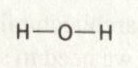Chemistry
In chemistry, we deal a lot with elements and how they are constructed. We often spend time making diagrams for molecules. Like H2O has a diagram that looks
like:
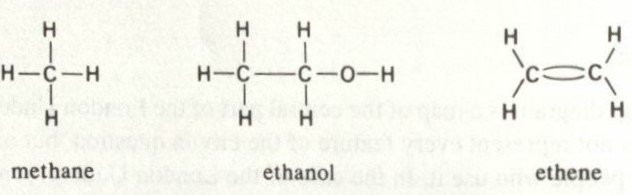
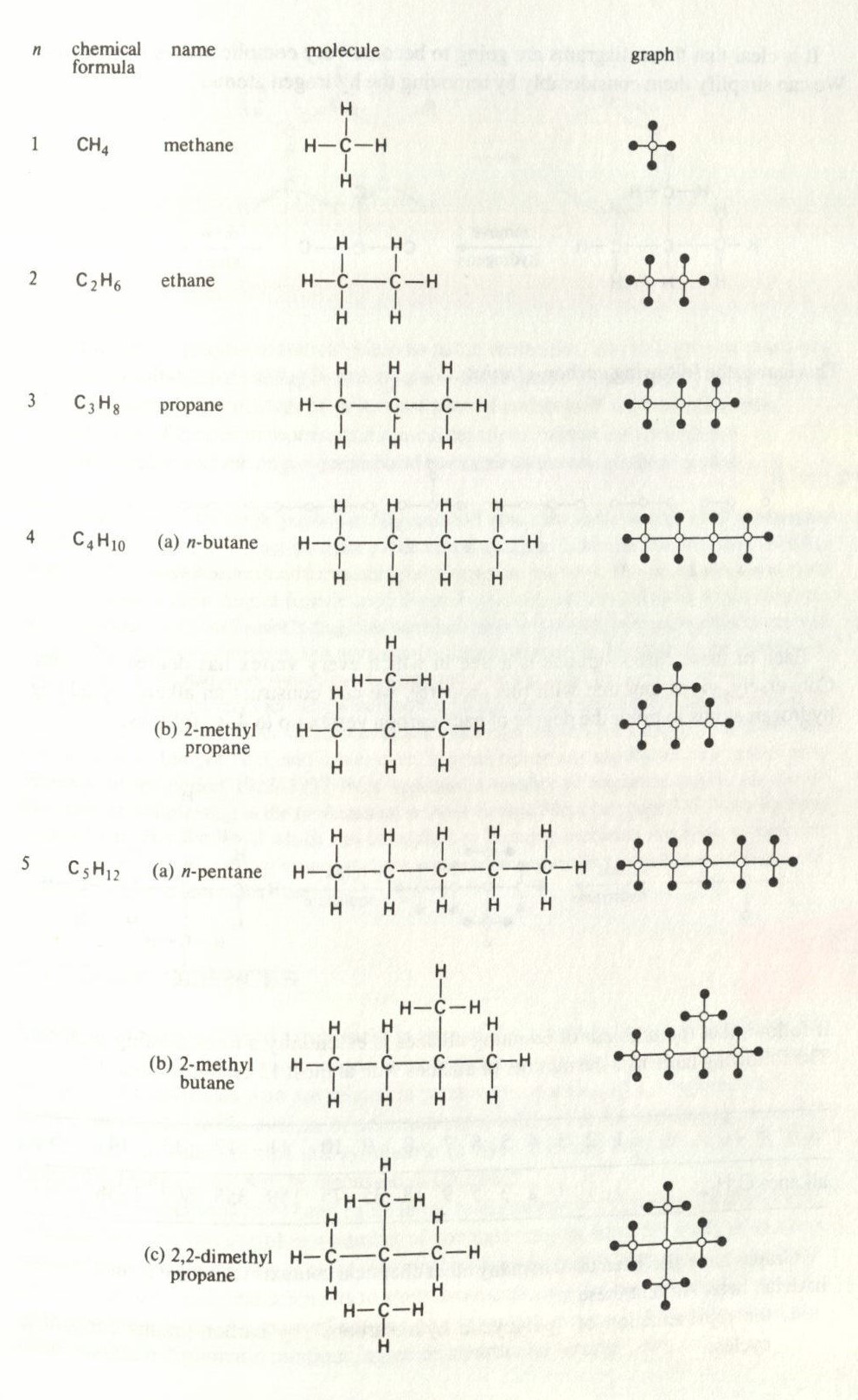
While methane, ethanol, and ethene have diagrams that look like:
These diagrams can be drawn as graphs, usually denoting specific elements with specific symbols (Wilson & Watkins, 1990)
.
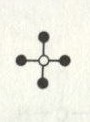
If we take methane and leave the carbon element empty, we would get a graph that looked like:
Graphs in this way are useful for finding isomers (molecules with the same chemical formula but different chemical properties). It also helps chemists to
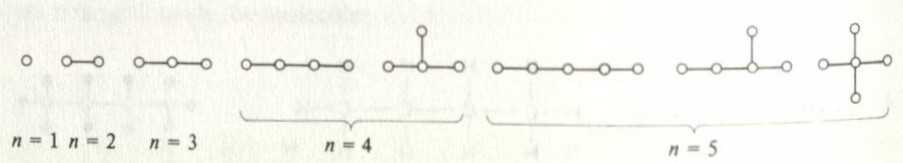
count the alkanes of CnH2n+2, which are the simplest organic molecules. Below is a chart for small values of n.
As we can see, as n gets large, it becomes very complicated. These graphs can then be simplified to just the carbon atoms, like: